Difference between revisions of "Talk:Equipment/Vinyl Cutter"
Joe Design (Talk | contribs) (→Have fun.) |
Joe Design (Talk | contribs) (→*Hi, Just to share a use for the Vinyl cutter in producing accurate and safe etching of copper.) |
||
| Line 4: | Line 4: | ||
Having cut and weeded the vinyl I applied it to cleaned and degreased copper. In this case, a copper plumbing pipe.<br /> | Having cut and weeded the vinyl I applied it to cleaned and degreased copper. In this case, a copper plumbing pipe.<br /> | ||
I applied the vinyl to the copper using the clear low tack tape to align the pattern.<br /> | I applied the vinyl to the copper using the clear low tack tape to align the pattern.<br /> | ||
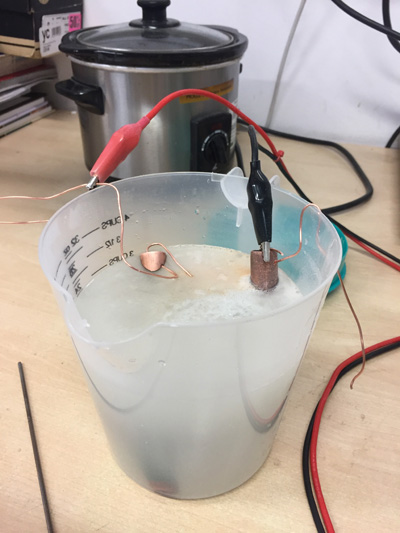
| − | I then removed the low tack tape and lowered the tube into my salt solution. This solution is made up of sea salt and warm water. You | + | I then removed the low tack tape and lowered the tube into my salt solution. This solution is made up of sea salt and warm water. You dissolve the salt until it will not take any more salt, then remove excess salt from the bottom by decanting the solution.<br /> |
Then I put another tube of the same size in the solution. '''Make sure these poles do not touch throughout the process'''<br /> | Then I put another tube of the same size in the solution. '''Make sure these poles do not touch throughout the process'''<br /> | ||
The anode from the charge circuit is connected to the piece to be etched. This is where the oxidisation will occur and the metal will give off electrons to the solution.<br /> | The anode from the charge circuit is connected to the piece to be etched. This is where the oxidisation will occur and the metal will give off electrons to the solution.<br /> | ||
Revision as of 15:21, 18 December 2017
Vinyl cutter in producing accurate and safe etching of copper
Having cut and weeded the vinyl I applied it to cleaned and degreased copper. In this case, a copper plumbing pipe.
I applied the vinyl to the copper using the clear low tack tape to align the pattern.
I then removed the low tack tape and lowered the tube into my salt solution. This solution is made up of sea salt and warm water. You dissolve the salt until it will not take any more salt, then remove excess salt from the bottom by decanting the solution.
Then I put another tube of the same size in the solution. Make sure these poles do not touch throughout the process
The anode from the charge circuit is connected to the piece to be etched. This is where the oxidisation will occur and the metal will give off electrons to the solution.
Then I connected the cathode to the other pipe.
A charge was then applied at around 2amps/2V for 20 minutes.
You will see bubbles at the cathode which will be hydrogen from the water and the solution will turn reddish brown.
The salt solution can be used again and again after settling and decanting the copper oxide off.